The quality and use of communication in PCa decision aids

Men newly diagnosed with localised prostate cancer are facing difficult decisions regarding their treatment. They need to choose from a range of treatment options (e.g., surgery, external beam radiotherapy, brachytherapy, or active surveillance), which have equivalent survival outcomes but differ in the risk of adverse events (1,2).
In the process of shared decision making, decision aids (web-based tools) provide information about treatment options and associated risks of side effects, and help patients get to know their values and preferences (3). Today, there are many patient decision aids available for prostate cancer patients, and according to a large Cochrane review, they seem to be effective (4). However, what is their quality? And to which extent do decision aids pay attention to communication features?
Large-scale systematic review
To answer these questions, a group of Dutch (health communication) scholars recently performed a large-scale systematic review to assess the quality and use of communication in currently available decision aids for patients with localised prostate cancer. They systematically searched through academic databases such as EMBASE or MEDLINE to collect decision aids that were, for instance, part of interventions in randomised controlled trials. In addition, they performed a thorough search through Google, since patients are more likely to find their information and decision aids in this environment (5). Eventually, they identified 19 international decision aids, of which 11 originated from North America and eight from Europe. The majority of the aids (12) were web-based tools, and the year of publication ranged from 2007-2018.
IPDAS checklist
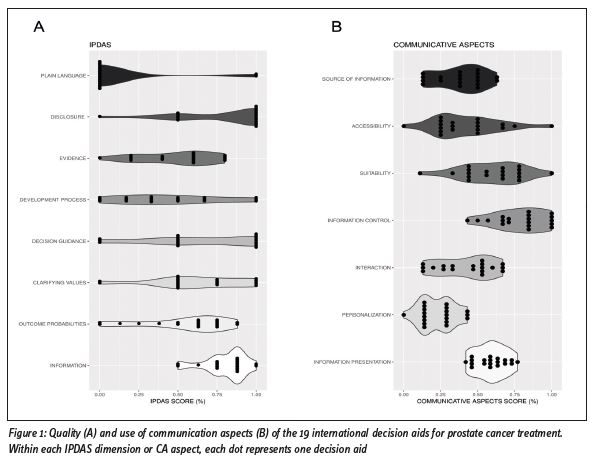
The authors first assessed the quality of the tools by using the validated International Patient Decision Aids Standards (IPDAS) checklist (6), which covers a variety of quality dimensions, ranging from evidence-based information about treatment options and outcome probabilities to decision guidance and development process. The authors found that the quality varied greatly across the decision aids, with many failing to comply with all components of the IPDAS criteria (mean IPDAS score = 59%, range = 36%-84%). This is also shown in Figure 1A, in which there is large variability for many IPDAS dimensions. Many aids did not adhere to good practice guidance on the presentation of outcome probabilities associated with treatment options, and lacked substantial information regarding the development process and readability levels of the aids.
Communicative Aspects checklist
After the quality assessment, the authors further reviewed the decision aids regarding their use of communication for which they developed the Communicative Aspects (CA) checklist (7,8). This tumour-independent checklist consists of 76 items divided into seven aspects: Information Presentation (e.g., how risks and uncertainties were communicated), Personalisation (e.g., how treatment information was tailored to patient characteristics), Interaction (e.g., how patients’ personal values and preferences were clarified), Information Control (e.g., how patients had control over access to and amount of information), Accessibility (e.g., whether the tool was easily accessible), Suitability (e.g., how suitable and understandable the content was) and finally Source of Information (e.g., whether and how the source of treatment information was given).
 Results
Results
The authors observed substantial variations in use of communication in decision aids (Figure 1B), with a mean CA score of 51% (range 32%-64%). Most importantly, few aids used visuals to communicate outcome probabilities, and none of them were personalised in terms of communicating the likelihood of experiencing treatment side effects. Furthermore, only a minority of the aids used interactive exercises to elicit patients’ values and preferences, and most tools had biased cross tables to compare the pros and cons of treatment options. The authors also found some issues with the suitability and accessibility of information in the aids that may hinder the uptake of decision aids in daily clinical practice.
Conclusion
What can we learn from this? According to the authors, this review demonstrates the variability among currently available decision aids for localised prostate cancer treatment, and shows that both their quality and use of communication can be improved. The authors recommend urologists who are using or developing decision aids to focus on personalisation techniques, such as communicating individualised risks of treatment side effects or tailoring the amount of treatment information to patient characteristics and preferences. Other possible improvements are the inclusion of interaction exercises for clarifying patients’ preferences and values, and using both text and visualisations for communicating statistical information. These suggestions are also relevant for clinicians outside of prostate cancer who are facing similar complex and time-consuming clinical counselling scenarios with their patients.
References
1. Hamdy FC, Donovan JL, Lane JA, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424. doi:10.1056/NEJMoa1606220
2. Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. J Urol. 2017;375(15):1425-1437. doi:10.1016/j.juro.2017.02.004
3. Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: Multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
4. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi:10.1002/14651858. CD001431.pub5
5. van Eenbergen MCHJ, Vromans RD, Boll D, et al. Changes in internet use and wishes of cancer survivors: A comparison between 2005 and 2017. Cancer. 2019;126(2):408-415. doi:10.1002/cncr.32524
6. Elwyn G, O’Connor AM, Bennett C, et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi). PLoS One. 2009;4(3):e4705. doi:10.1371/journal. pone.0004705
7. Vromans RD, van Eenbergen MC, Pauws SC, et al. Communicative aspects of decision aids for localized prostate cancer treatment – A systematic review. Urol Oncol Semin Orig Investig. 2019;37(7):409-429. doi:10.1016/j.urolonc.2019.04.005
8. Vromans R, Tenfelde K, Pauws S, et al. Assessing the quality and communicative aspects of patient decision aids for early-stage breast cancer treatment: A systematic review. Breast Cancer Res Treat. 2019;178(1):1-15. doi:10.1007/s10549-019-05351-4 Source: Communicative aspects of decision aids for localized prostate cancer treatment: A systematic review. Vromans RD, van Eenbergen MC, Pauws SC, Geleijnse G, van der Poel HG, van de Poll-Franse LV, Krahmer EJ. Urol Oncol Semin Orig Investig. 2019 Apr;37(7):409-429. https://doi.org/10.1016/j. urolonc.2019.04.005
__________________________________________________________________________________________________________
Ruben Daniël Vromans, MA MPhil, PhD Candidate, Dept. of Communication and Cognition, Tilburg University (NL), r.d.vromans@tilburguniversity.edu

